세계에서 가장 가벼운 '금' 개발 A new form of real gold, almost as light as air(VIDEO)
스위스 취리히연방공대(ETH) 연구팀
동물 부드러운 털, 작은 꽃잎 위에도 놓여져
가느다란 주형(틀) 위에 금 입자 굳히는 방식 적용
공기 만큼 가벼운 금. 꽃잎과 카푸치노 거품 위에도 얹을 수 있을 만큼 가볍다. - 스위스 취리히연방공대 제공
edited by kcontents
케이콘텐츠 편집
카푸치노 거품 위에 살포시 앉을 수 있을 만큼 ‘가벼운 금’이 새로 나왔다. 이 금은 동물의 부드러운 털이나 작은 꽃잎 위에도 거뜬히 내려앉는다.
스위스 취리히연방공대(ETH) 연구팀은 아주 가느다란 주형(틀) 위에 금 입자를 굳히는 방식으로 가벼운 금을 만드는 데 성공했다고 ‘어드밴스드 머티리얼스’ 11월 23일 자 온라인 판에 발표했다.
연구팀은 먼저 우유에 들어있는 단백질을 끓여 수 나노미터(nm·10억 분의 1m) 굵기의 선으로 이뤄진 틀을 만들었다. 이 틀에 금 용액을 넣자 용액이 틀에 엉겨 붙으면서 젤 같이 물렁한 덩어리를 이루는 것으로 나타났다. 금과 단백질이 뭉친 덩어리를 꺼낸 뒤에는 이산화탄소로 조심스레 말렸다.
그 결과 내부에 구멍이 많아 98%가 비어 있는 금덩이가 탄생했다. 색과 광택은 금과 동일하지만, 손으로 늘릴 수 있을 정도로 부드러운 등 물성은 기존 금과 달랐다.
게다가 단백질 틀에 붙는 금 입자의 크기를 조절하면 금덩이의 색도 바꿀 수 있다. 연구팀이 금 입자를 더 작은 크기로 만들자 노란 금덩이 대신 검붉은 금덩이가 나왔다. 빛을 흡수하는 능력과 반사 능력이 바뀌었기 때문이다.
구스타프 니스트르 연구원은 “틀에서 제작한 구조를 파괴하지 않고 말리는 것이 이번 연구의 가장 큰 도전과제였다”며 “이번에 만든 금을 시계와 장신구는 물론 촉매 등 다양한 화학반응에 이용할 수 있을 것”이라고 말했다. 동아사이언스 신선미 기자 vamie@donga.com |
A new form of real gold, almost as light as air
By: Fabio Bergamin | 9 comments
Researchers at ETH Zurich have created a new type of foam made of real gold. It is the lightest form ever produced of the precious metal: a thousand times lighter than its conventional form and yet it is nearly impossible to tell the difference with the naked eye. There are many possible applications.

Even when it seems unbelievable: this is a genuine photograph, in which nothing has
been faked. The 20 carats gold foam is lighter than milk foam. (Photo: Gustav Nyström
and Raffaele Mezzenga / ETH Zurich)
A nugget of real 20 carats gold, so light that it does not sink in a cappuccino, floating instead on the milk foam – what sounds unbelievable has actually been accomplished by researchers from ETH Zurich. Scientists led by Raffaele Mezzenga, Professor of Food and Soft Materials, have produced a new kind of foam out of gold, a three-dimensional mesh of gold that consists mostly of pores. It is the lightest gold nugget ever created. "The so-called aerogel is a thousand times lighter than conventional gold alloys. It is lighter than water and almost as light as air," says Mezzenga.
The new gold form can hardly be differentiated from conventional gold with the naked eye – the aerogel even has a metallic shine. But in contrast to its conventional form, it is soft and malleable by hand. It consists of 98 parts air and only two parts of solid material. Of this solid material, more than four-fifths are gold and less than one-fifth is milk protein fibrils. This corresponds to around 20 carat gold.
Drying process a challenge
The scientists created the porous material by first heating milk proteins to produce nanometre-fine protein fibres, so-called amyloid fibrils, which they then placed in a solution of gold salt. The protein fibres interlaced themselves into a basic structure along which the gold simultaneously crystallised into small particles. This resulted in a gel-like gold fibre network.
"One of the big challenges was how to dry this fine network without destroying it," explains Gustav Nyström, postdoc in Mezzenga's group and first author of the corresponding study in the journal Advanced Materials. As air drying could damage the fine gold structure, the scientists opted for a gentle and laborious drying process using carbon dioxide. They did so in an interdisciplinary effort assisted by researchers in the group of Marco Mazzotti, Professor of Process Engineering.
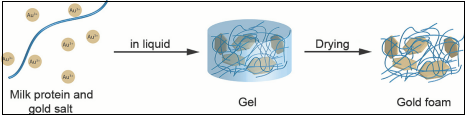
Milk protein filaments and gold salt are the starting materials for the gold foam.
(Ilustration: Nyström G et al. Advanced Materials 2015)
Dark-red gold
A foam of amyloid protein filaments
without gold (above), with gold
microparticles (middle) and gold
nanoparticles (below). (Photo: Nyström
G et al. Advanced Materials 2015)
The method chosen, in which the gold particles are crystallised directly during manufacture of the aerogel protein structure (and not, for example, added to an existing scaffold) is new. The method's biggest advantage is that it makes it easy to obtain a homogeneous gold aerogel, perfectly mimicking gold alloys.
The manufacturing technique also offers scientists numerous possibilities to deliberately influence the properties of gold in a simple manner. " The optical properties of gold depend strongly on the size and shape of the gold particles," says Nyström. "Therefore we can even change the colour of the material. When we change the reaction conditions in order that the gold doesn't crystallise into microparticles but rather smaller nanoparticles, it results in a dark-red gold." By this means, the scientists can influence not only the colour, but also other optical properties such as absorption and reflection.
The new material could be used in many of the applications where gold is currently being used, says Mezzenga. The substance's properties, including its lighter weight, smaller material requirement and porous structure, have their advantages. Applications in watches and jewellery are only one possibility. Another application demonstrated by the scientists is chemical catalysis: since the highly porous material has a huge surface, chemical reactions that depend on the presence of gold can be run in a very efficient manner. The material could also be used in applications where light is absorbed or reflected. Finally, the scientists have also shown how it becomes possible to manufacture pressure sensors with it. "At normal atmospheric pressure the individual gold particles in the material do not touch, and the gold aerogel does not conduct electricity," explains Mezzenga. "But when the pressure is increased, the material gets compressed and the particles begin to touch, making the material conductive."
케이콘텐츠
kcontents
"from past to future"
데일리건설뉴스 construction news
콘페이퍼 conpaper










